Questions - Chemistry
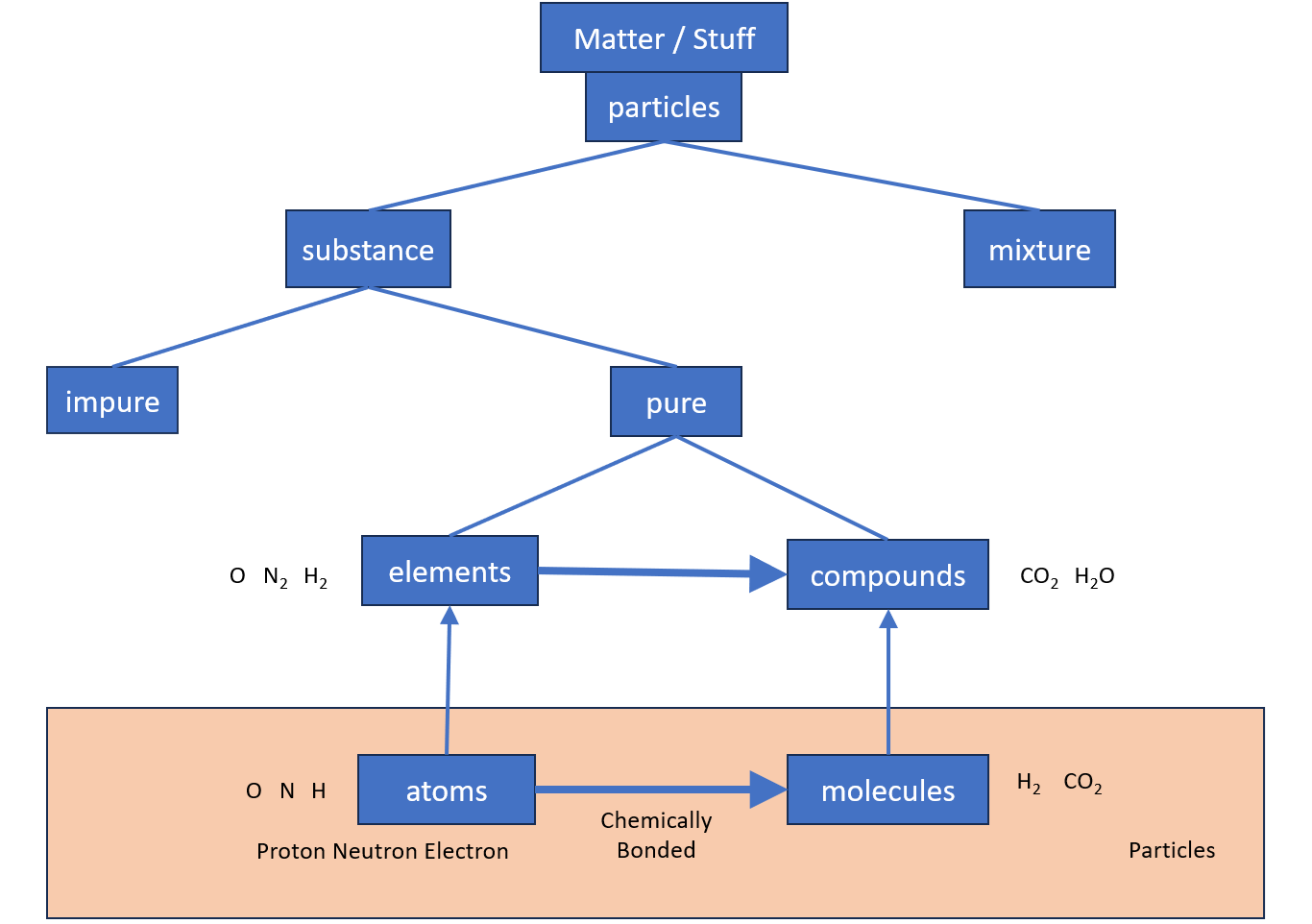 |
1) What is Matter ?
Anything that has mass and takes up space is recognised as matter. All stuff/materials are matter.
2) What is Mass and how can it be measured ?
Mass is a measure of the amount of matter in an object. Mass is usually measured in grams (g) or kilograms (kg).
It can be measured using a "top pan balance".
3) What is a Particle ?
Matter is made up of particles.
A particle can be an atoms, molecules or ions.
Particles are very small and cannot be seen with a microscope
4) Do particles get bigger when they are heated ?
No.
5) What is a Vacuum ?
A space with no particles of matter in it.
6) What is Particle Theory ?
Also known as the Particle Model.
All matter is made up of particles.
Matter can exist in 3 states: solid, liquid or gas
Matter can change state.
This explains how particles are arranged in solids, liquids and gases and what properties they have
At a certain temperature the particles have enough energy to break the strong forces holding them together (melting, solid to liquid)
At a certain temperature the particles have enough energy to break the weak forces holding them close together (boiling, liquid to gas)
7) Can you give some physical properties of a Solid ?
It is a solid when the temperature of the material is lower than the melting point.
hard/strong, fixed shape, fixed volume, high density, low energy
cannot be compressed easily
8) Can you give some physical properties of a Liquid ?
It is a liquid when the temperature of the material is between the melting point and boiling point.
can flow/be poured, fixed volume, medium density
cannot be compressed easily
shape changes to fill bottom of a container
9) Do liquids diffuse faster than gases when at the same temperature ?
No.
10) Can you explain what causes pressure in a liquid ?
The liquid particles move and collide with each other and with the side of the container.
The average force of these particles (over the area of the sides of the container) is called the pressure.
11) What is Brownian Motion ?
This is the name used to describe when particles move randomly in random directions and collide.
12) When does brownian motion occur ?
It occurs in liquids.
Solid particles suspended in a liquid.
Gas particles suspended in a air.
example - pollen grains in water
13) Why does brownian motion occur ?
This is caused by density fluctuations in the fluid.
The kinetic energy of the fluid molecules.
14) Can you give some physical properties of a Gas ?
It is a gas when the temperature of the material is greater than the boiling point.
does not keep the same shape or volume
can diffuse, low density, high energy
can be compressed
gases have the same shape and volume as the container they are in
15) What does Permeable mean ?
This is the name given to a material that allows liquids and gases to pass through it or soak into it.
eg Sandstone
16) What does Impermeable mean ?
This is the name given to a material that does not allow liquids or gases to pass through it.
eg Marble
17) What is Diffusion ?
This is the process where particles of one gas (or liquid) substance spread out in between the particles of another gas (or liquid) substance.
Diffusion in gases is much faster than diffusion in liquids
The particles spread out and move from an area of high concentration to an area of low concentration.
The following three factors affect the rate of diffusion: temperature, particle size and concentration
18) What are the different ways that state can be changed ?
Melting - solid to liquid, increase in energy, particles break free from fixed position
Freezing - liquid to solid, decrease in energy
Boiling - liquid to gas, increase in energy, fill container (also includes Evaporation)
Condensation - gas to liquid, decrease in energy - happens at any temperature below the boiling point
Sublimation - solid to gas, particles break free from fixed position, increase in energy
Deposition - gas to solid, decrease in energy
19) Is Boiling and Evaporation the same thing and explain why ?
They are both processes that change a liquid to a gas but they are not the same thing.
Boiling is faster, only occurs are boiling temperature and occurs everywhere. The state slowly changes to gas creating bubbles that rise to the surface to escape.
Evaporation only occurs on the surface and can happen at any temperature.
20) What is Latent Heat ?
This is the extra heat needed to cause a substance to melt.
The stronger the intermolecular forces between the particles the more latent heat is needed to melt the substance.
21) What is Density ?
This is a measure of how many particles there are in a given space.
Gases are usually less dense than solids of the same substance.
22) What is the formula that connects density, mass and volume ?
Density = Mass / Volume
23) What is the density of 14g of gold that has a volume of 10 cm3 ?
density = 14/10 = 1.4 g/cm3
24) What is the mass of 10cm3 of liquid that has a density of 1 g/cm3 ?
mass = density x volume
mass = 10 x 1 = 10g
25) What is the volume of 55g of pure water, if water has a density of 1 g/cm3
volume = mass / density
volume = 55 / 1 = 55 cm3
26) When cooking oil is added to water, will it float or sink ?
The cooking oil has a density of 0.9 g/cm3.
Cooking oil will float because it has a lower density than water.
27) What piece of equipment is used to measure temperature ?
Thermometer
28) What is Conduction ?
Conductors are materials that allow heat to easily pass through it (metals)
Insulators are materials that are poor conductors (non-metals, gases, plastic)
29) What is an Atom ?
The atom is the smallest unit of matter.
It is composed of three sub-atomic particles: the proton, the neutron, and the electron.
30) What is Ionisation ?
This is the removal of an electron from an atom.
When this happens it creates a positive ion.
31) What is an Element ?
Made of only one type of atom. Everything in the periodic table is an element.
O - one Oxygen atom
H2 - two Hydrogen atoms
32) What is the Periodic Table ?
Every element has a name and a symbol (one or two letters)
Depending on the temperature every element can be changed into solid, liquid or gas.
The periodic table provides a list of all the different elements
The table is divided into groups (which go down) and periods (which go across)
For example the first group are all soft, shiny metals
All the elements in a column/group have similar properties
The properties of the elements change as you go down the column.
Elements at the bottom of the column/group act more violently than those at the top.
33) How many elements are there in the periodic table ?
118 in total. 95 metals (including 7 metalloids), 22 non-metals
34) What is a Metalloid ?
An element intermediate in properties between the typical metals and non-metals.
35) Can you list the 7 metalliods ?
Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium and Polonium
36) What are the Symbols for the following elements ?
Sulpur - S
Iron - Fe
Hydrogen - H
Carbon - C
Magnesium - Mg
Aluminium - Al
Sodium - Na
Chlorine - Cl
Calcium - Ca
Zinc - Zn
Oxygen - O
Copper - Cu
Potassium - K
Silver - Ag
Gold - Au
37) What is a Molecule ?
Made of two or more atoms chemically bonded, due to a chemical reaction. They may or may not be the same type of atom.
H2 - two Hydrogen atoms
O2 - two Oxygen atoms
CO2 - different atoms
38) What is a Compound ?
Compounds are molecules that have different types of atoms
All compounds are molecules.
CO2
39) What is the difference between Molecules and Compounds ?
Molecules can be elements or compounds
40) What is the difference between Molecules and Elements ?
Elements are made up of only one type of atom.
Molecules can be made up of different types of atoms.
41) Give an example of a Molecule that is not a Compound ?
Molecules are elements when they are only made up of one type of atom
H2 - is not a compound
42) Give an example of a Compound that is also a Molecule ?
All compounds are molecules.
CO2
43) What is the difference between Physical Change and Chemical Change ?
Physical Change - its molecular composition stays the same. Affects only physical properties (shape, size, density). Can be easily reversed.
Chemical Change - its molecular composition is changed. Affects both physical and chemical properties. These are irreversible.
44) Do all Compounds have a Chemical Formula ?
Yes. The formula contains the symbols of the elements that make up the compound.
In a chemical reaction you start with "Reactants" and you end up with "Products".
45) What is a Chemical Reaction ?
This is a process in which one or more substances are converted into one or more different substances.
46) How do you Create a Compound ?
All compounds are formed from chemical reactions.
Some of these chemical reactions occur naturally, for example water (H2O) and carbon dioxide (CO2).
Water Synthesis is a chemical reaction in which two molecules of hydrogen combine with one molecule of oxygen.
47) How do you Separate a Compound ?
You need a chemical reaction to separate any atoms that have been chemically bonded.
48) What is a Substance ?
It has the same properties all the way through.
Substances can be pure or impure.
49) What is a Pure Substance ?
A pure substance is made from just one type of element or one type of compound.
50) Is the air we breath a pure or impure substance ?
Impure because it contains different elements (nitrogen, oxygen) and compounds (cardon dioxide)
51) Is Salt a pure or impure substance ?
Pure because it only contains one type of compound (sodium chloride symbol - NaCl).
52) Can you describe these different types of substances ?
Base - a substance that reacts with an acid
Neutral - a substance that makes a solution with a PH=7
Salt - a substance made in a neutralisation reaction
Alkali - a substance that reacts with an acid that can dissolve in water to make a solution with a PH>7
Acid - a substance that contains hydrogen and can dissolve in water to make a solution with PH<7
53) What is a Mixture ?
Two or more different substances that have not been chemically bonded together.
In a mixture the different substances can be separated using a physical process.
A substance is not a mixture
For exampe iron and sulphur
54) Can you describe the following types of mixtures ?
Emulsion - Two liquids (one water based, one oil based)
Colloid - Different states of matter that are dispersed together
Foam - Gas bubbles trapped in a liquid
Gel - Liquid properties in a solid
Aerosol - Liquid or solid particles in a gas
55) How do you Create Mixtures ?
One way to create a mixture is to add a solid to a liquid and watch it Dissolve. For example salt in water.
The strong forces holding the particles together are broken down allowing the solid particles to mix with the liquid particles.
Solvent - The name given to the liquid substance that the solid is being dissolved into
Soluble - A solid substance is called soluble if it will dissolve
Insoluble - A solid substance is called insoluble if it will not dissolve
Solution - The name given to a mixture of a solid (solute) with a liquid (solvent) that does not separate out.
56) What does Concentration mean ?
The measure of the number of solvent particles in a given volume of solution.
57) What is the difference between Density and Concentration ?
The Main difference is that concentration refers to how much of a substance is present in a mixture.
Whereas density refers to the mass of a substance per unit volume.
58) What does the word Dissolve mean ?
The process where a solute in a gaseous, liquid, or solid phase dissolves in a solvent to form a solution
example - adding ammonium nitrate to water
example - adding salt to water
59) What is Water ?
It is the H2O compound in its liquid state.
60) Can gases dissolve in water ?
Yes
61) What is Ice ?
It is the H2O compound in its solid state.
62) When a compound changes from a solid to a liquid, the density normally decreases and the volume increases.
However H2O does the opposite.
63) When a compound changes from a liquid to a solid, does the density and volume increase or decrease ?
Density normally increases.
Volume normally decreases.
However H2O does the opposite.
64) Can you describe what happens when a solid dissolves ?
When something dissolves it does not disappear
There is no change in mass
If you evaporate off the liquid you will see the solid particles
65) How do you Separate Mixtures ?
If particles are not chemically bonded they can usually be separated.
There are a number of separation techniques (or physical methods) that can be used:
Filtration
This technique separates pieces of solid that are mixed with a liquid by pouring through filter paper.
Evaporation
This technique separates a soluble solid from a liquid (creating crystals)
Distillation
This technique separates a liquid from a solution (water can be separated from salty water) Uses evaporation and condensation to obtain a solvent from a solution
Chromatography
This technique separate different coloured liquids
66) Can you describe how you would separate rock salt which is a mixture of salt and sand.
Grind the rock and mix it with water.
The salt will dissolve because it is soluble.
The sand will fall to the bottom it is insoluble.
The sand could be seperated by using filtration using filter paper
The salt could then be separated by using evaporation
The water could be seperated by using condensation by cooling down the water vapour.
67) Some changes are called Reversible ?
These materials will change when heated and then return to how they were before, when cooled down.
For example salt in water
68) Some changes are called Irreversible ?
Bicarbonate of soda will dissolve in vinegar
69) What is the difference between Chloride and Chlorine ?
Chloride is formed from chlorine.
Chloride is what is created when Chlorine gains an electron and combines with other elements
70) Can you give some examples of chemical reactions ?
Sodium + Hydrogen = Sodium Hydride
Hydrogen + Oxygen = Water
Sodium + Hydrochloric Acid = Sodium Chloride + Hydrogen
Hydrogen + Carbon = Methane Gas
71) What is a Carbonate ?
A carbonate is a salt of carbonic acid,
Carbonates are compounds containing CO32 ions.
Carbonates react with dilute acids to form a salt, carbon dioxide, and water.
example - calcium carbonate + hydrochloric acid = calcium chloride + carbon dioxide + water
example - copper2 carbonate = copper2 oxide + carbon dioxide
72) What is Decomposition ?
Also called chemical breakdown.
The process of simplifying a single chemical entity (normal molecule, reaction intermediate, etc.) into two or more fragments.
73) What is Smelting ?
This is a process of applying heat and a chemical reducing agent to an ore to extract a desired base metal product.
74) What is a Monomer ?
Monomers are atoms or small molecules that bond together to form more complex structures such as polymers.
There are four main types of monomer, including sugars, amino acids, fatty acids, and nucleotides.
75) What does "Extrusive Igneous" mean ?
Extrusive igneous rocks are formed by magma that has erupted onto the surface as lava and then cooled quickly.
76) What does "Intrusive Igneous" mean ?
Intrusive igneous rocks are igneous rocks formed inside of Earth's crust. They are formed as magma slowly cools and solidifies underneath Earth's surface.
77) What are CFCs ?
Chlorofluorocarbons (CFCs) are nontoxic, nonflammable chemicals containing atoms of carbon, chlorine, and fluorine.
They are used in the manufacture of aerosol sprays, blowing agents for foams and packing materials, as solvents, and as refrigerants.
78) Can you describe some physical properties of Metals ?
Metals conduct electricity
Metals conduct energy
Metals are strong and tough
Metals are shiny when polished
Metals are sonorous
Metals are ductile
Metals have high densities
Metals have high melting and boiling points
79) Can you describe some physical properties of Non-Metals ?
Non-metals don't conduct electricity
Non-metals don't conduct energy (by heating well)
Non-metals are not strong or hard wearing
Non-metals are dull
Non-metals are brittle
Non-metals have low densities
Non-metals have low melting and boiling points
80) Can you describe some different types of polymers (or plastics)
nylon, polythene, PVC
81) What is Steel ?
Steel is an alloy of iron and carbon containing less than 2% carbon and 1% manganese and small amounts of silicon, phosphorus, sulphur and oxygen.
Steel is the world's most important engineering and construction material.
82) Why is Steel harder than pure Iron ?
Steel contains atoms of other elements as well as iron.
These atoms have different sizes to iron atoms, so they distort the layers of atoms in the pure iron.
This means that a greater force is required for the layers to slide over each other in steel.
83) What is Crude Oil ?
Crude oils is a mixture of chemicals called hydrocarbons.
These are chemicals that contain hydrogen and carbon only.
It made from ancient biomass, mainly plankton.
Crude oil straight out of the ground is not much use, as there are too many substances in it, all with different boiling points.
84) Can you describe some different types of ceramics ?
glass and porcelain
85) What are Composite Materials ?
These are materials that are made from two or more materials stuck together
eg - Fibre Glass (glass fibre and plastic)
eg - Concrete - sand, gravel and cement
86) What are Chemicals ?
This is the name given to substances that are used in a chemical reaction
Chemicals is another word for substances
The chemicals you start with are called the reactants
The chemicals you finish with are called the products
Chemical formulas are used to indicate what is happening in a chemical reaction
These chemical formulas are called symbol equations.
87) Can you describe what happens in a chemical reaction ?
atoms are not changed or destroyed
atoms move around
the mass does not change (the products weigh the same as the reactants)
there is always a transfer of energy to or from the surroundings
88) Can you give the names of different types of chemical reactions
Oxidation - this is when a substance reacts with oxygon releasing energy (heat and light)
Combustion is an oxidation reaction
Rusting is an oxidation reaction (iron + oxygon = iron oxide)
Thermal Decomposition - this is when a substance is heated and breaks down
Neutralisation -
89) What is an Exothermic Reaction ?
This is a chemical reaction that transfers energy to the surroundings
rusting, camp fires, water evaporation, water + acid reacting, nuclear fusion
90) What is an Endothermic Reaction ?
This is a chemical reaction that takes energy from the surroundings
water to ice, cooking an egg, photosynthesis, dissolving salt in water, baking bread
91) What is the name of the scale used to measure how Acid or Alkaline a substance is ?
The PH scale (from 0 to 14)
Substances with a PH less than 7 are called acids (hydrochloric acid)
Substances with a PH more than 7 are called alkaline (sodium hydroxide)
Substances with a PH equal to 7 are considered neutral (water)
92) What is a control variable ?
Something in an experiment you keep the same.
93) What is an independent variable ?
The thing being changed.
94) What is a dependent variable ?
The thing that is measured.
© 2025 Better Solutions Limited. All Rights Reserved. © 2025 Better Solutions Limited TopPrevNext